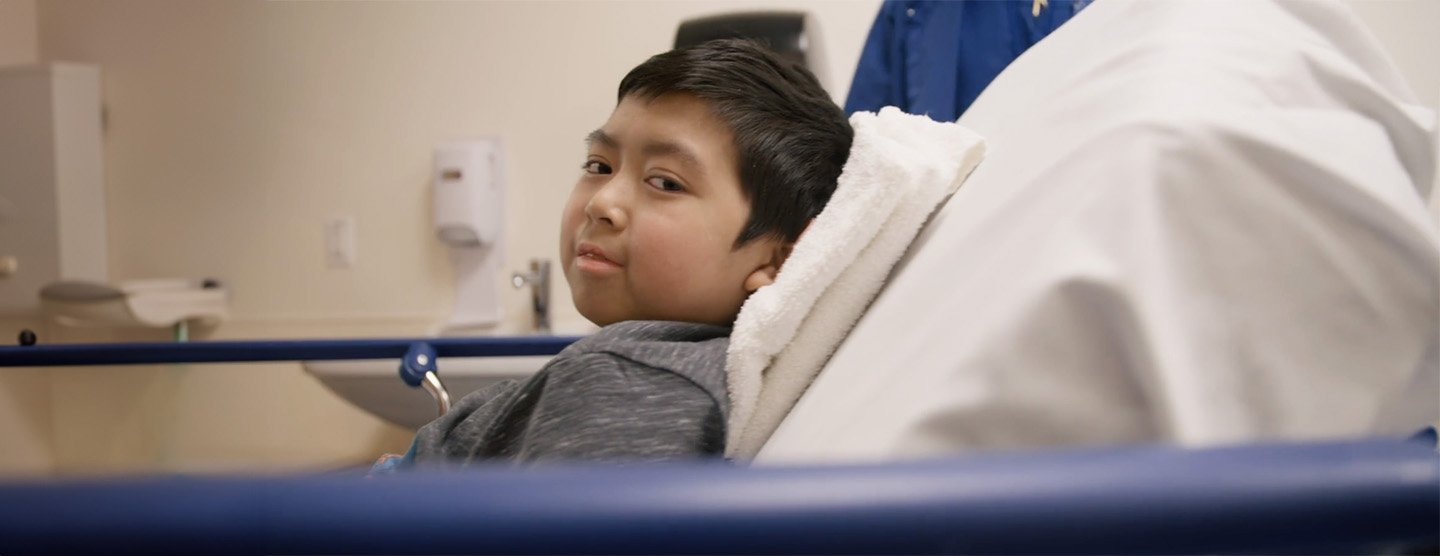
Blood & Marrow Transplant (BMT) Clinic
Bone marrow or stem cell transplantation can be lifesaving for children with certain cancers, blood disorders and immune system disorders. But the procedures that must be followed before and after a transplant are complicated and demanding. At the Pediatric Blood & Marrow Transplant (BMT) Clinic, we are dedicated to preparing kids for a BMT transplant and providing the long-term follow-up care they need afterward.
Our team of transplant specialists evaluates whether your child is a transplant candidate and, if so, works to get your whole family ready for the BMT process. Because transplantation severely compromises immune function, we monitor our pediatric BMT patients closely. After the procedure, we see them at least once a week to check for infections and other complications and to assess their immune system's recovery.
This high-quality follow-up care is one reason our patients' survival rates one year after transplant are among the highest in the nation.
Expanding treatment options
Stem cells for transplant may be taken from the patient's blood or bone marrow (called an autologous transplant) or, more commonly, from a donor (an allogeneic transplant). Ideally, donors are family members who are "matched" to the patient, meaning certain markers in their immune cells are compatible with those of the patient. This reduces the risk that the patient's body will reject the transplant. However, our experts are skilled in using partially matched related donors (called haploidentical donors) when fully matched donors aren't available.
We also offer CAR T-cell therapy, a relatively new option for treating certain types of cancer when traditional approaches fail. In essence, the therapy supercharges the patient's immune system to help it recognize and destroy their cancer cells. We were the first medical center in California to offer CAR T-cell therapy to eligible children.
Our team is continually working to improve BMT care by conducting laboratory research as well as clinical trials. By joining a clinical trial, patients may be able to receive promising experimental treatments before they're available to the general public. If your child is a candidate for a clinical trial, the care team will discuss the potential risks and benefits with you and your family.
Awards & recognition
-

Best in Northern California for cancer care
-

Ranked among the nation's best in 11 specialties
-

Designated an early phase clinical trials core site by the Children's Oncology Group
-

Accredited by the Foundation for Accreditation of Cellular Therapy
Our locations (3)
Highlights of our cancer care

Leading-edge treatments
We're among the first to offer new treatment options, as soon as they're proven safe and effective. In addition, patients can access promising experimental therapies by participating in one of our many clinical trials.
Precision medicine
High-risk tumors are analyzed with the UCSF500 Cancer Gene Panel, a state-of-the-art test that identifies mutations in the tumor's DNA. The results can indicate the best treatment course and, in some cases, clarify the exact type of cancer.
Seamless care, near and far
Dedicated patient navigators help families with managing referrals, insurance concerns and appointment planning. We work with patients and referring doctors well beyond the San Francisco Bay Area, thanks to our comprehensive telehealth system.
Thorough follow-up
Our Pediatric Survivorship Program provides expert care to address any medical, psychological or social issues that arise after treatment is complete. The team also ensures smooth transitions to adult care providers as our patients leave childhood behind.

Our team
-

Anurag K. Agrawal
MD
Pediatric hematologist-oncologist -

Julia Chu
MD, MPH
Pediatric hematologist-oncologist -

Morton J. Cowan
MD
Pediatric immunologist and bone marrow transplant specialist -

Jasmeen S. Dara
MD, MS
Pediatric immunologist and allergist -

Christopher Dvorak
MD
Pediatric hematologist-oncologist -

Robert E. Goldsby
MD
Pediatric hematologist-oncologist -

Michelle L. Hermiston
MD, PhD
Pediatric hematologist-oncologist -

Christine Higham
MD
Pediatric hematologist-oncologist -

James Huang
MD
Pediatric hematologist -

Sandhya Kharbanda
MD
Pediatric hematologist-oncologist -

Nahal Lalefar
MD
Pediatric hematologist-oncologist -

Gabriel Salinas Cisneros
MD
Pediatric hematologist-oncologist and bone marrow transplant specialist -

Kristin A. Shimano
MD
Pediatric hematologist-oncologist -

William Temple
MD
Pediatric hematologist-oncologist and bone marrow transplant specialist -

Lena Winestone
MD, MPH
Pediatric hematologist-oncologist -

Mark Walters
MD
Pediatric hematologist-oncologist -

Mara Bailey-Olson
CPNP
Pediatric nurse practitioner -

Sophia Chao
NP
Pediatric nurse practitioner -

Janelle Facchino
NP, MS
Pediatric nurse practitioner -

Brianne Gebhardt
NP, BSN
Nurse practitioner -

Sara O'Kane
NP
Pediatric nurse practitioner
Plan your visit
What to Bring
- Photo I.D.
- Health insurance card
- Insurance authorization, if required
- Doctor's referral, if required
- Recent test results related to your child's condition
- List of medications, including dosages, plus any your child is allergic to
- List of questions you may have
- Device or paper for taking notes
Related clinics (6)
 7
7
Hematology Clinic

Primary Immune Regulatory Disorder (PIRD) Clinic
Support services
Clinical trials
A Multicenter Access and Distribution Protocol for Unlicensed Cryopreserved Cord Blood Units (C...
The primary objective of this access and distribution protocol is to examine the incidence of neutrophil recovery of ≥500/mm3 after cord blood transplantation in a multi-institution setting using CBUs that are not FDA licensed.
Recruiting
More about this studyAutologous Gene Therapy for Artemis-Deficient SCID
Patient survival status and (if applicable) cause of death will be recorded to assess overall survival.
Recruiting
More about this studyA Feasibility Study Using CLINIMACS® for Alpha/Beta T-Cell Depletion in Stem Cell Transplant
The cumulative incidence of Grade III-IV acute GVHD at Day 100 will be summarized by incidence curves. GVHD evaluations will be performed using standard criteria.37 Patients with graft rejection will be censored.
Recruiting
More about this studyCaloric Restriction and Activity to Reduce Chemoresistance in B-ALL
To compare the rate of MRD >=0.01% at end of induction between experimental arm and control arm
Recruiting
More about this studyConditioning SCID Infants Diagnosed Early
Humoral immune reconstitution by 2 years post HCT, defined by specific antibody response to tetanus toxoid. Criteria for evaluation of humoral immune response are the following: - Donor T cell chimerism ≥50% - B cell count...
Recruiting
More about this studyA Trial Comparing Unrelated Donor BMT With IST for Pediatric and Young Adult Patients With Seve...
The median time to failure or death will be compared on the two arms using the log-rank test. Failure of IST is defined as the date that a second definitive therapy was recommended (BMT, second course of ATG) and failure of BMT de...
Recruiting
More about this studyTransplantation of Clustered Regularly Interspaced Short Palindromic Repeats Modified Hematopoi...
The adverse event rate will be summarized using descriptive statistics, together with 95% confidence intervals where appropriate. No formal statistical hypothesis testing will be performed. Adverse events defined: failure of engra...
Recruiting
More about this studyTagraxofusp in Pediatric Patients With Relapsed or Refractory CD123 Expressing Hematologic Mali...
The incidence of dose limiting toxicity (DLT) will be measured at different dose levels.
Recruiting
More about this studyUsing Ultrasound Elastography to Predict Development of Hepatic Sinusoidal Obstruction Syndrome
To define a threshold and quantify the sensitivity and specificity of US SWE for risk stratification of patients into three categories as defined by the EBMTC adult and pediatric criteria: no SOS, mild to moderate SOS, and severe ...
Recruiting
More about this studyEBV-specific Cytotoxic T-lymphocytes (CTLs) for Refractory EBV Infection
Patients will be monitored for adverse events related to the infusion of EBV CTLs
Recruiting
More about this studyOur research initiatives
Stress-free visits
Accommodations. Admissions. Procedure prep. Get the info you need for smoother hospital stays.
Prepare for your child's stay